$25.00
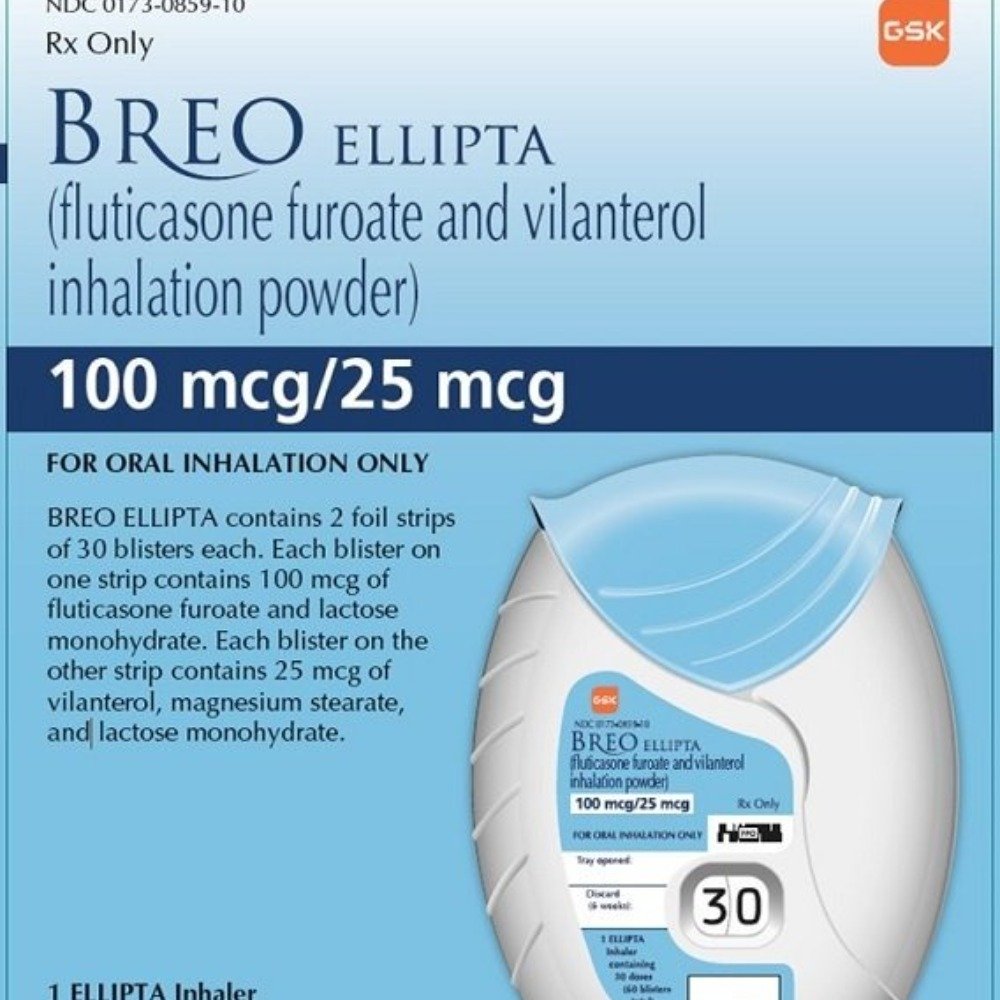
Breo Ellipta 100/25 mcg
(Fluticasone Furoate 100 mcg / Vilanterol 25 mcg)
Description:
Breo Ellipta 100/25 mcg is a once-daily inhalation medication used to treat chronic obstructive pulmonary disease (COPD) and asthma in adults. It combines fluticasone furoate, a corticosteroid that reduces inflammation in the airways, with vilanterol, a long-acting beta-agonist (LABA) that helps to relax the muscles around the airways. This combination works together to improve lung function, reduce symptoms, and help prevent exacerbations associated with asthma and COPD.
Breo Ellipta is designed for long-term maintenance treatment. It is not intended for the immediate relief of acute bronchospasm. The combination of anti-inflammatory action from fluticasone furoate and bronchodilation from vilanterol helps provide 24-hour relief from asthma and COPD symptoms such as wheezing, shortness of breath, coughing, and tightness in the chest.
Key Features:
Reviews
There are no reviews yet.