$25.00
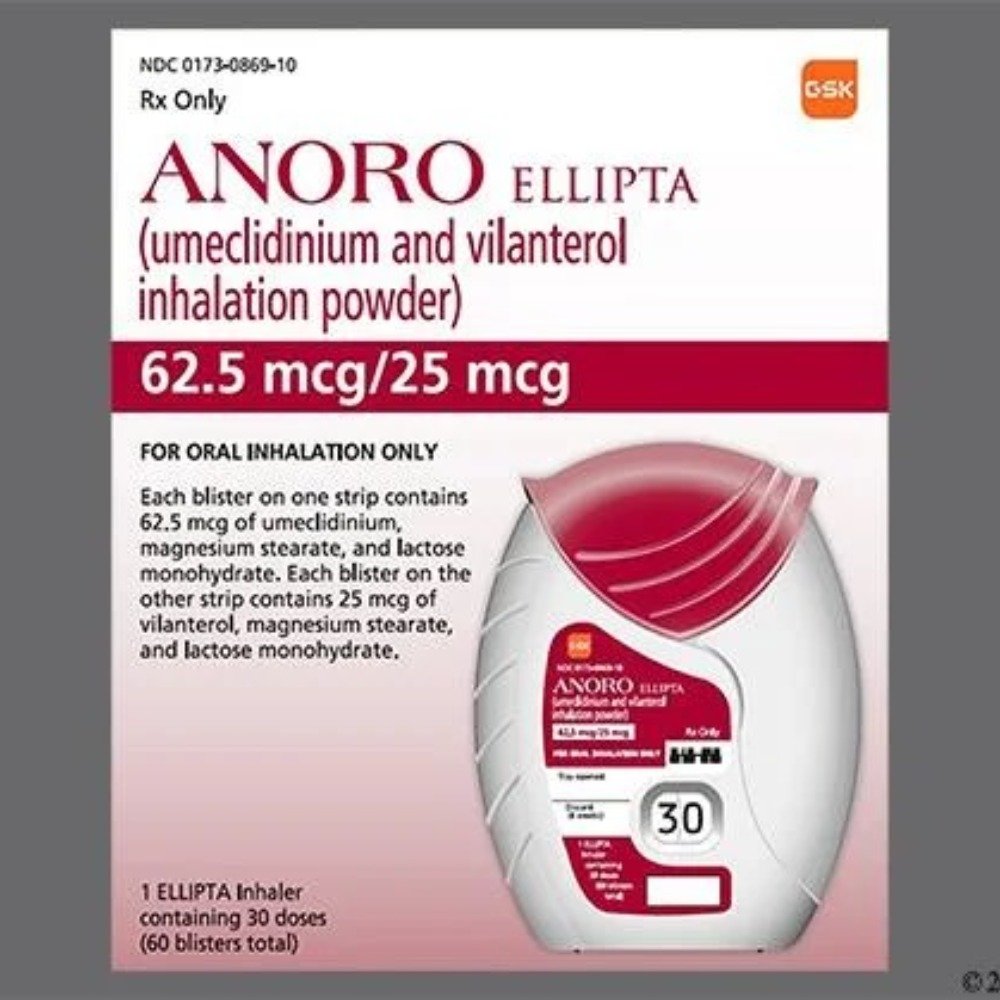
Anoro Ellipta
(Umeclidinium 62.5mcg / Vilanterol 25mcg)
Description:
Anoro Ellipta is a combination inhalation medication used for the long-term management of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. It contains two active ingredients: umeclidinium (a long-acting muscarinic antagonist, or LAMA) and vilanterol (a long-acting beta-agonist, or LABA). Together, these medications help relax the muscles around the airways to prevent spasms and improve airflow in the lungs, making it easier to breathe.
Anoro Ellipta is prescribed as a once-daily inhaler for patients with COPD who need maintenance treatment to control symptoms such as wheezing, shortness of breath, and coughing. By combining a muscarinic antagonist with a beta-agonist, Anoro Ellipta provides a dual mechanism of action that helps reduce airway constriction and improve lung function for individuals suffering from chronic respiratory conditions.
Key Features:
Reviews
There are no reviews yet.